Oxybenzone has similar effects on all organisms:
Toxicity
Endocrine Disruption
Feminization and
Sterility
What You Must Know
The sunscreen chemical that is in over 60% of sunscreens is rapidly absorbed, and according to the CDC, 97% of Americans are contaminated with oxybenzone (that can be found in your urine).
The exact properties that make it a good photoprotector also make it a ubiquitous environmental contaminant, bioaccumulated up the food chain with us at the top.
Properties:
Most lipophilic (meaning it loves fat —> also doesn’t break down in water, rather accumulates in fatty tissues and is metabolized)
Photostable: doesn’t break down in sunlight
Phototoxic: adverse effects are exacerbated by light, particularly UV radiation.
It produces free radicals as it absorbs sunlight (a byproduct of the chemical reaction that occurs to convert UV radiation into heat energy on your skin)
1/4 of people will develop a sunburn-like reaction upon exposure to the sun.
Most rapidly absorbed in the skin, where it causes widespread endocrine disrupting effects, mimicking the hormone estrogen.
It increases estrogen and leads to sterility in many species.
Hormonal effects have been detected since 2001.
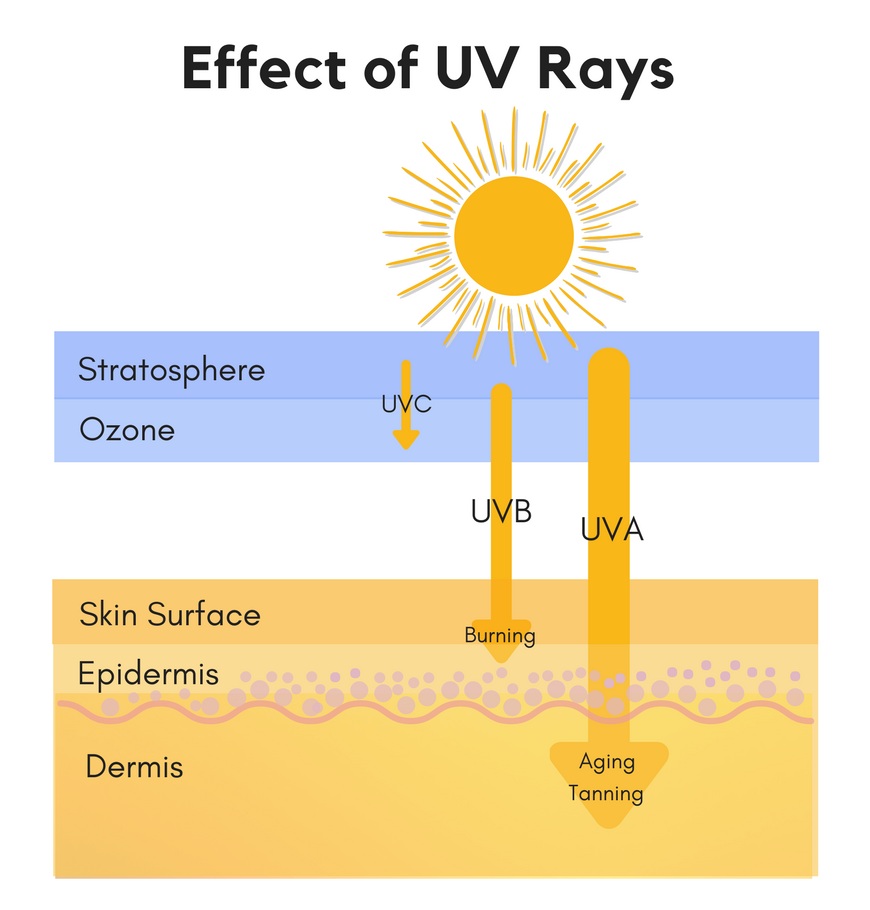
Absorption
Oxybenzone is absorbed by humans within 20 minutes after application.
There, it can be detected in your urine for days.
It accumulates in your fatty tissues (as it is highly lipophilic)
There, as we try to break down this toxin, it is metabolized into more toxic byproducts, and has been shown to mimic the hormone estrogen, leading to sterility, reproductive diseases, developmental disorders, and neurological problems.
Once metabolized, it is excreted, where it goes to a wastewater treatment plant (WWTP), and cannot be broken down. Oftentimes, it emerges from wastewater effluent (what comes out after it’s been treated)
However, children do not yet have the metabolism to break down oxybenzone, and was deemed “unsuitable for children” in 2006.
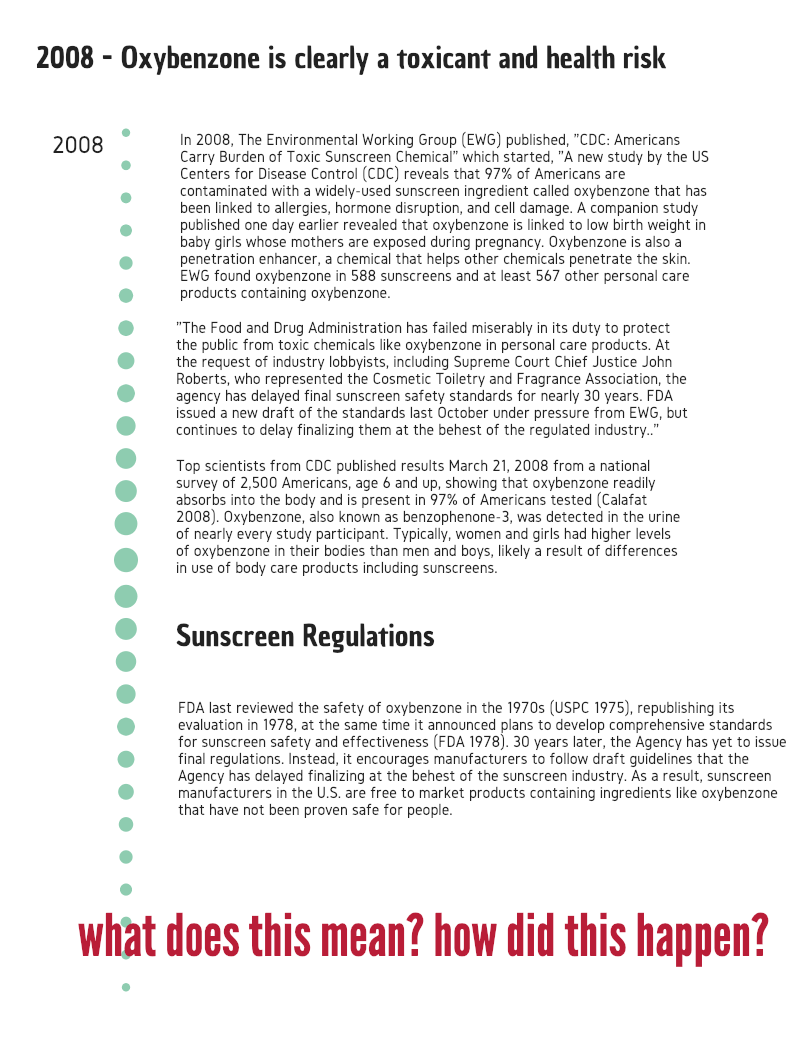
The Environmental Working Group (EWG) urged the FDA to look at oxybenzone in 2008, citing numerous studies proving negative human health impacts and ecological impacts. Notoriously, they failed to take action.
For over 15 years, the toxic effects of oxybenzone have been known.
Sunscreen companies are already reformulating and taking oxybenzone out.
But you’re probably still using it.